Research
Eosinophils in colorectal cancer progression
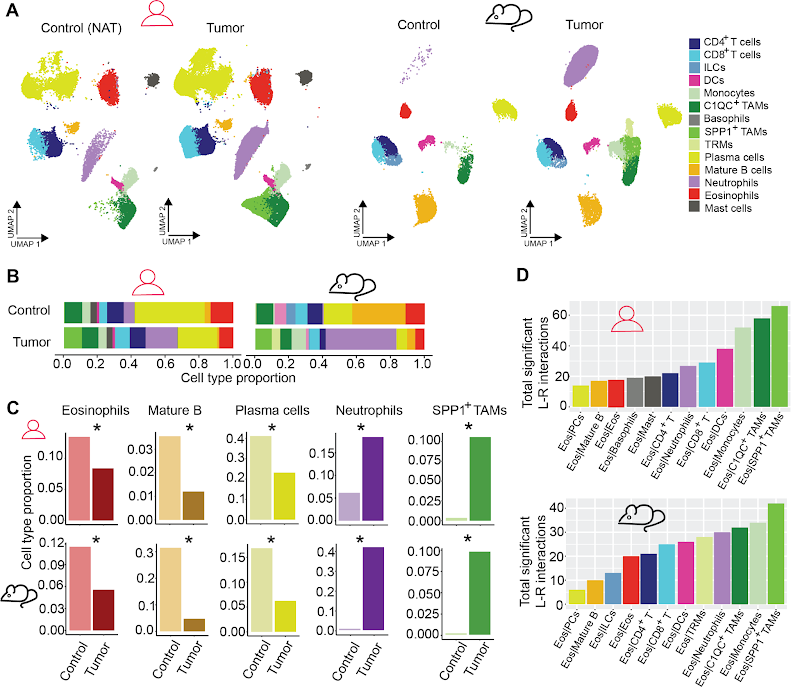
Under homeostatic conditions, eosinophils are heterogeneously distributed in our organisms. One particulary abundant reservoir is the gastrointestinal (GI) tract and spatial location makes them potentially relevant during the development of GI malignancies, and studies have shown that high eosinophil infiltrations in colorectal cancer correlate with an improved prognosis. However, the molecular mechanisms of how eosinophils influence tumor progression are not well understood. Therefore, we are studying the role of eosinophils with murine models of early- and late-stage CRC, in parallel with patient biopsies. We use a combination of diverse technologies and methods like eosinophil depleted mouse strains, fluorescence-activated cell sorting (FACS), immune fluorescence imaging, single cell RNA sequencing, spatial transriptomics and in-vitro organoid culture to shed light on the underlying mechanisms.
Lung Project Immunology
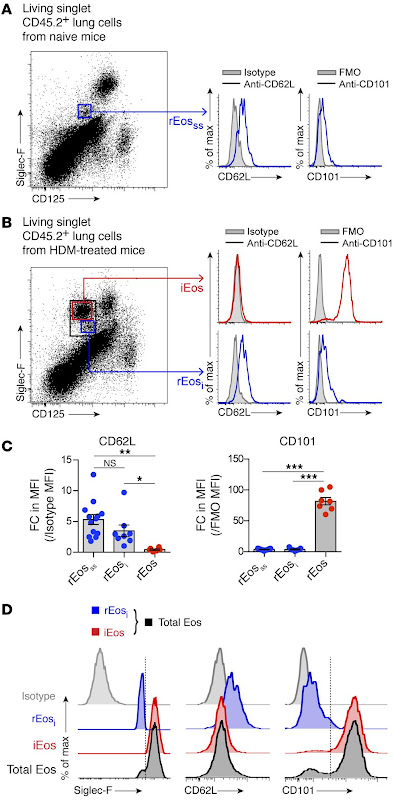
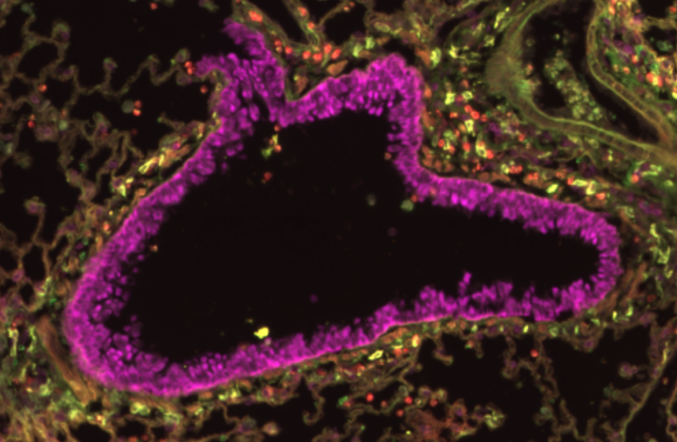
Airway diseases (e.g. allergic asthma, COPD) affect hundreds of millions of people worldwide and pose a significant medical burden. Bronchodilators are the mainline treatment for these diseases but this treatment is ineffective in severe cases of disease. This is largely due to the immune system driving severe inflammation in the lungs, causing tissue fibrosis and mucus plugging. Consequently, therapies that block the immune response have been developed to treat these airway diseases, achieving some success but also met with treatment resistance and side effects. Notably, eosinophils play a critical role in driving immune pathology in the lungs during allergen-induced asthma, with disease severity strongly linked to eosinophil numbers. Thus, there is a huge interest to identify targets to block eosinophil responses in the lungs to treat airway disease pathology. Intriguingly, lung eosinophils are separated into two distinct subsets; there is a resident eosinophil population and an IL-5 dependent inflammatory eosinophil population (recruited during airway inflammation). Whilst there has been promise in targeting the inflammatory eosinophil population with anti-IL-5 biologics to treat airway inflammation, other studies have highlighted that all lung eosinophils are IL-5 dependent, questioning the current paradigm of eosinophil heterogeneity in the lung.Here, we aimed to dissect the heterogeneity of lung eosinophils using novel single-cell RNA sequencing (scRNA-seq), flow cytometry and immunofluorescence imaging. Using these approaches we can identify how inflammatory eosinophil responses are formed and how we can target this to alleviate eosinophil-mediated inflammation in the context of airway diseases (e.g. allergic asthma).
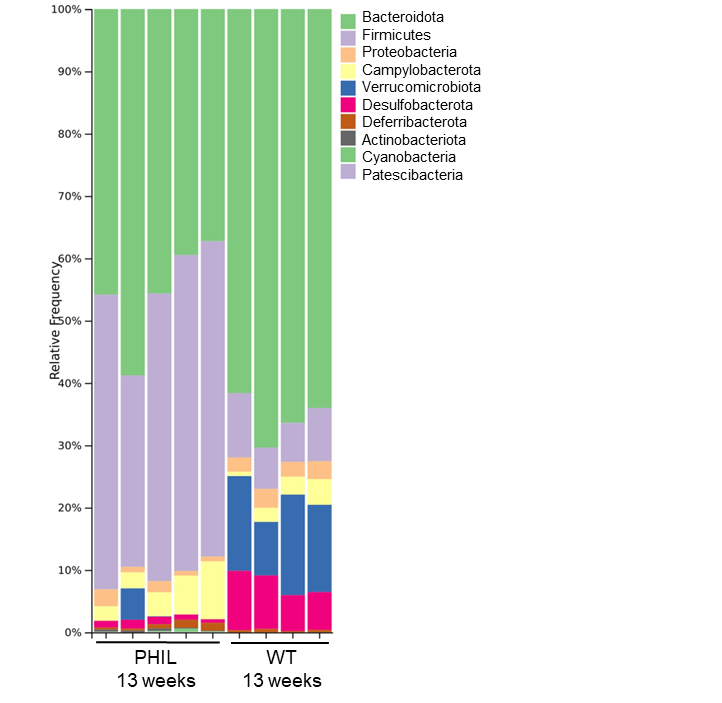
Homeostasis Project
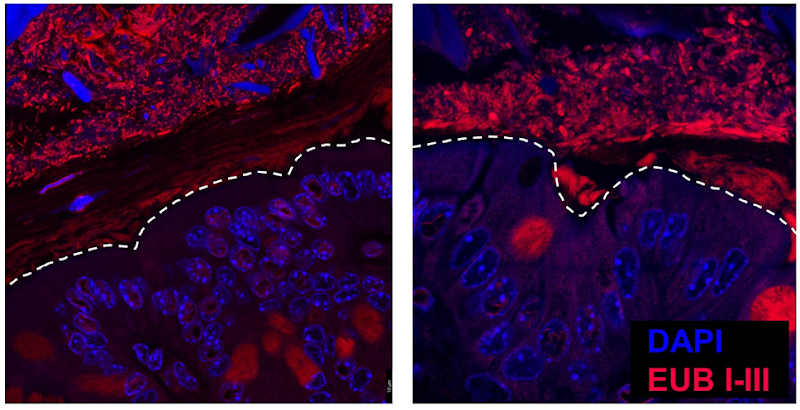
By uncovering how eosinophils influence gut health and immune development, we aim to better understand the foundations of lifelong health and disease prevention. In the gastrointestinal tract, eosinophils are crucial in maintaining a healthy balance between the host and its resident bacteria. We are particularly interested in how eosinophils influence the gut microbiota, driving tissue development and gut barrier integrity. Our research explores how eosinophils shape microbial communities and modulate immune responses to maintain intestinal homeostasis. Using genetically modified mouse models, we investigate how the absence of eosinophils impacts microbiome composition, tissue architecture, and long-term immune function. We are using advanced immunofluorescence and multiplexed 3D imaging to dissect eosinophil spatial localisation within the tissues, revealing how their positioning supports host–microbial interactions. Alongside this, we apply single-cell RNA sequencing, microbial profiling, and functional immunological assays to explore the molecular mechanisms guiding eosinophil–bacteria cross-talk. By uncovering how eosinophils influence the gut microbiome and immune development, we aim to better understand the foundations of lifelong health and disease prevention.